Improve Antibody Half-Life Measurements
If you’re developing a therapeutic molecule, the half-life of your molecule in circulation is an essential factor to consider. A longer half-life means patients don’t need to be dosed as often, therapies are more likely to be efficacious, and therefore, the treatment is more likely to be approved by regulatory agencies like the FDA.
Which molecules have a long half-life in blood?
Monoclonal antibodies, specifically those of the IgG class, experience significantly longer half-lives than other molecules. Additionally, IgGs have a high degree of target specificity. Both of these characteristics make IgGs desirable therapeutic molecules (Fig 1).
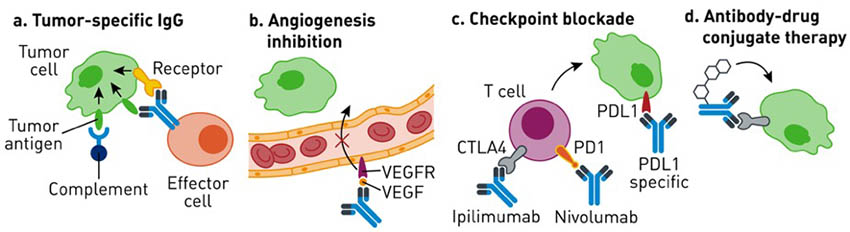 Fig 1. Examples of monoclonal antibodies used in cancer therapy. IgGs are versatile molecules that generate a therapeutic response through a variety of mechanisms: eliciting immune effector functions (A), blocking ligand-receptor interactions (B, C), or delivering targeted chemical payload (D). Adapted from Weiner GJ, 2015. |
IgG half-life is primarily regulated by binding to the neonatal Fc Receptor, FcRn, through the IgG’s constant region, Fc. This Fc-FcRn binding occurs in the acidic lysosome of endothelial cells and rescues IgG from cellular degradation pathways. Intact IgG is then released back into the bloodstream at neutral pH. Fc- and albumin-conjugates also have long half-lives because they, too, bind FcRn and are protected similarly (Fig 2).
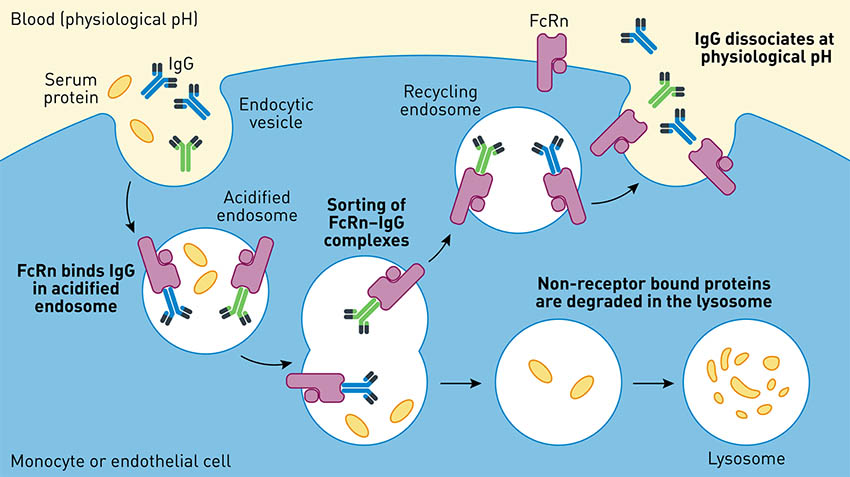 Fig 2. IgGs experience high serum half-life due to the protective FcRn recycling pathway. Adapted from Roopenian et al., 2007. |
Why should I measure the half-life of my therapeutic?
Antibody half-life can be affected by pH, by modifications made to variable regions, by choice of linkers, or by the type of payload attached, as in the case of antibody-drug conjugates. Because of this, you may need to test the half-life of several variant molecules before moving forward with a particular candidate.
How do I monitor half-life?
The kinetics (Kd) of human FcRn interactions can be quantified quickly and relatively inexpensively using in vitro methods like cellular binding assays and Surface Plasmon Resonance-based approaches. Eventually, clinically-relevant pharmacokinetic values such as half-life will need to be characterized. Typically, in vivo PK measurements are done by injecting the candidate molecule intravenously into an animal model and measuring levels of the molecule in the serum over several hours and days (Fig 3).
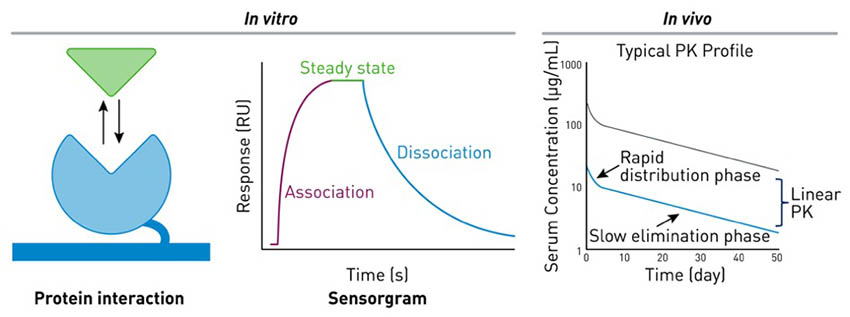 Fig 3. Typical in vitro and in vivo methods to measure antibody half-life. Adapted from www.sprpages.nl and Kamath et al., 2016. |
What kinds of in vivo models help model half-life for human therapeutics?
Standard or wild type mice can be relatively inexpensive in vivo models. However, human IgG (and by extension, human Fc-conjugates) binds to mouse FcRn through different amino acid residues resulting in PK data that doesn’t correlate well with human data. Thus, when standard mice are used, half-life outcomes can be highly variable, and clinically-relevant data is not produced.
Alternatively, non-human primate (NHP) models very accurately model human PK due to the species similarity in Fc-FcRn binding. However, NHP models carry significant financial costs as well as significant ethical concerns for testing.
One possible solution to improve clinical relevance while also reducing dependence on NHP models is to use genetically modified mouse models. In these models, such as B6.Cg-Fcgrttm1Dcr Tg(FCGRT)32Dcr/DcrJ (FcRn Tg32, 014565) and B6.Cg-Fcgrttm1Dcr Tg(CAG-FCGRT)276Dcr/DcrJ (FcRn Tg276, 004919), the mouse FcRn is removed in exchange for the human FcRn. This allows for the appropriate species Fc-FcRn binding affinities that standard mice fail to model.
Which humanized FcRn model is right for my therapeutic?
When half-life measurements are compared for clinically approved therapeutic IgGs, the values in humanized FcRn mice, particularly for FcRn Tg 32, correlate strikingly well to human data (Fig 4A).
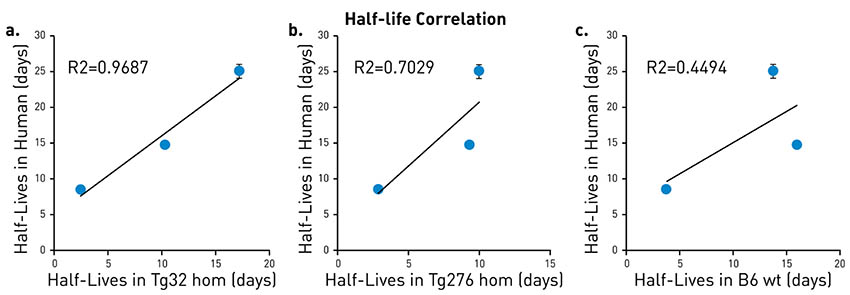 Fig 4. Belatacept, Ipilimumab, and Pembrolizumab half-life values were measured in FcRn Tg32 (A), FcRn Tg 276 (B), and wildtype mice (C) and compared to known human data. FcRn Tg32 generated the most clinically predictive data, while wildtype mice failed to predict human data. |
While it shows an intermediate correlation to human data (Fig 4B), the strength of the FcRn Tg276 model lies in its ability to allow fine distinctions in half-life between several candidate molecules (Fig 5).
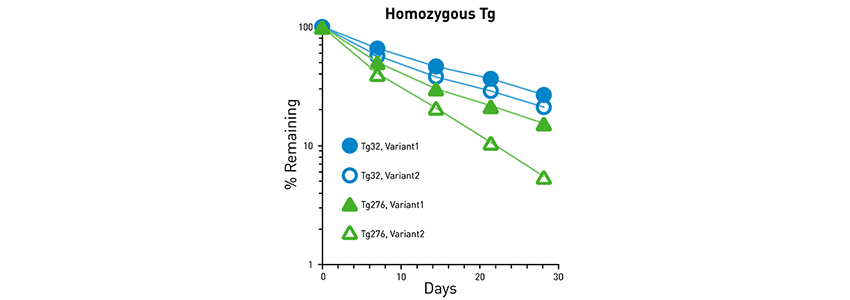 Fig 5. FcRn Tg276 allows distinction between several variant therapeutic IgGs. Variant 1 shows longer half-life compared to Variant 2, which is more easily discerned using the FcRn Tg276 model compared to the FcRn Tg32 model. |
If you are concerned your therapeutic may generate anti-therapeutic antibodies in the mouse host, immunodeficient versions of these humanized FcRn models are available. Importantly, the immunodeficiency in these models does not alter the half-life correlation data demonstrated in the original immunocompetent versions.
Who provides services to test half-life of my therapeutic for me?
The humanized FcRn mouse models were created and published at The Jackson Laboratory by Dr. Derry Roopenian. As part of JAX’s mission to empower the global biomedical community to discover solutions for human disease, we offer Therapeutic Antibody Half-Life Evaluation Services using these unique and clinically relevant humanized FcRn mouse models to researchers developing novel IgG, Fc-, or albumin-based therapeutics. Contact the JAX Technical Information Scientist Team to learn more.
Recommended Resources
Therapeutic Antibody Evaluation Services
Blog: Revealing antibody half-life stability using FcRn mouse models from JAX
Webinar: De-Risk Therapeutic Antibody Drug Development Using Humanized FcRn Mice
References
Roopenian DC; Christianson GJ; Sproule TJ; Brown AC; Akilesh S; Jung N; Petkova S; Avanessian L; Choi EY; Shaffer DJ; Eden PA; Anderson CL. 2003. The MHC class I-like IgG receptor controls perinatal IgG transport, IgG homeostasis, and fate of IgG-Fc-coupled drugs. J Immunol 170(7):3528-33 [PMID: 12646614]
Chaudhury C; Mehnaz S; Robinson JM; Hayton WL; Pearl DK; Roopenian DC; Anderson CL. 2003. The major histocompatibility complex-related Fc receptor for IgG (FcRn) binds albumin and prolongs its lifespan. J Exp Med 197(3):315-22 [PMID: 12566415]
Kamath AV. 2016. Translational pharmacokinetics and pharmacodynamics of monoclonal antibodies. Drug Discov Today Technol. 21-22: 75-83. [PMID: 27978991]
Weiner GJJ. 2015. Building better monoclonal antibody-based therapeutics. Nat Rev Cancer. 15(6); 361-70. [PMID: PMC4491443]