Revealing Antibody Characteristics with JAX Services
Introduction
Drug developers regularly exploit the natural ability of B cells to create hundreds of thousands of antibodies and select the ones specific to their target (Singh S., 2018). Depending on the site of interaction, antibodies can block or activate the target protein, favor its clearance, or induce an immune response against cells expressing the target. Moreover, antibodies can be conjugated to drugs or toxins to increase their local concentration at specific sites.
As compared to other proteins that have a serum half-life of a few hours, antibodies are very stable in circulation. Immunoglobulin G (IgG), the most commonly used isotype for therapeutic development, has an approximate half-life of 21 days. The mechanism behind IgG's extended half-life lies in the interaction between IgG and the neonatal Fc receptor (FcRn) (Roopenian et al., 2003; Challa et al., 2014). Cells lining the blood vessels internalize IgG and shuttle it to the lysosome. The low pH in acidified endosomes causes IgG to bind with FcRn. The IgG: FcRn complexes are subsequently recycled away from the lysosomal degradative pathway to the cell membrane, where, at neutral pH, IgG dissociates from FcRn, releasing IgG back into circulation.
The IgG:FcRn interaction is mediated by the Fc portion of the antibody. The Fc fragment is also the main target of protein engineering efforts aimed at optimizing the activity of antibodies (Wang X., 2017). For example, many blocking antibodies require silencing mutations to avoid the recruitment of immune effector cells, while other antibodies are modified to activate specific cell types (i.e., NK cells or macrophages). Crucially, some of these mutations might interfere with the formation of the IgG:FcRn complex, thereby reducing the stability of the therapeutic antibody candidate.
A major challenge facing researchers who are developing therapeutic antibodies is assessing their half-life to select the most stable variants and determine the dosage for clinical trials. Given this complex biology underlying the stability of antibodies in vivo, how can researchers get accurate antibody clearance data, saving both time and resources?
How do I predict antibody half-life?
Measuring in vivo half-life of therapeutic candidate molecules is often overlooked because, until a few years ago, the available animal models had significant limitations to test the half-life of human antibodies accurately. Standard rodent models are relatively inexpensive and easy to work with. However, mouse and rat FcRn do not bind human IgG with the same kinetics as human FcRn, therefore leading to unreliable half-life data (Ober et al., 2001). While non-human primates can predict with reasonable accuracy the half-life of human antibodies in patients, their use for screening at early stages in drug development is not a feasible option due to both ethical and practical reasons.
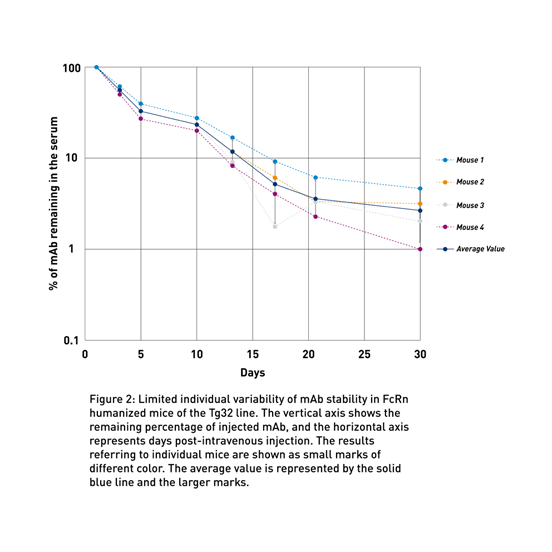
To address the lack of preclinical models to predict human therapeutic half-life accurately, JAX scientist Dr. Derry Roopenian developed mice expressing transgenic human FCGRT, the gene that encodes FcRn and lacking the endogenous murine gene (Proetzel and Roopenian, 2014). These “Tg32” and “Tg276” FcRn humanized mouse models can be used to predict the PK of IgG antibodies in humans with an accuracy comparable to non-human primates (Avery LB, 2016; Fig. 1a and Fig. 1b). Furthermore, as shown in figure 2 using the Tg32 model as an example, the variability between the mice is minimal, enabling a small number of animals per study.
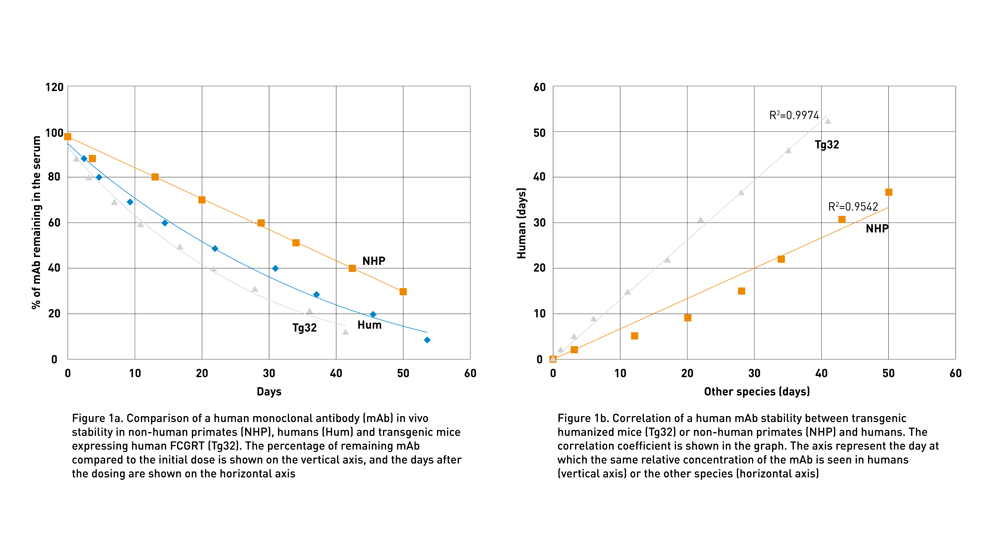
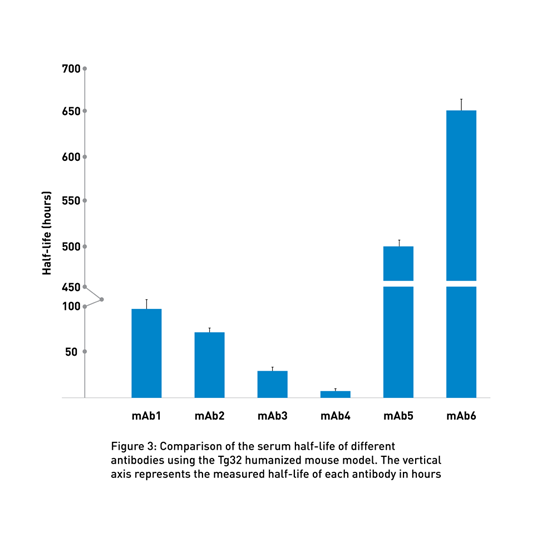
By using these transgenic lines, the PK experts at the JAX Therapeutic Evaluation Services routinely compares the half-life of different client-supplied molecules to identify the most promising candidates (Fig 3 and seminar by Greg Christianson, JAX).
How can I identify the right antibody variant?
Fc engineering results in multiple variants of the same antibody, each with slightly different in vivo half-life and potentially different effector functions. Due to their similar structure, it is often difficult to identify the variant with the most extended half-life even using the humanized Tg32 model (Fig 4a).
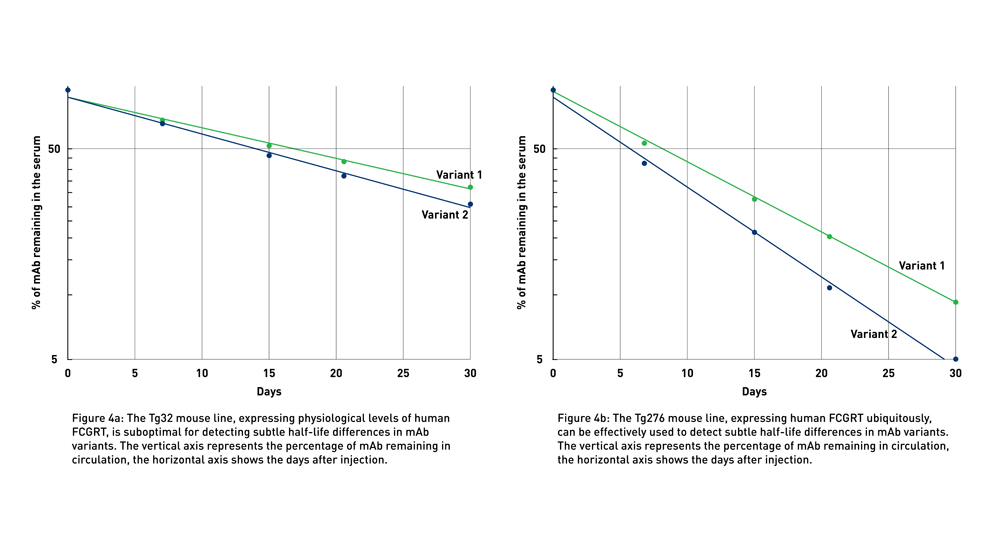
JAX Therapeutic Antibody Evaluation Services rely on the use of human FcRn Tg276 mice (004919), which ubiquitously express the human gene at high levels in all tissues (Latvala et al., 2017). Although the levels and pattern of expression of the transgene in Tg276 mice do not recapitulate the physiological expression of FCGRT in humans, these animals exacerbate even small differences in antibody half-lives and, therefore, represent the optimal tool to detect the smallest differences between similar monoclonal antibodies (mAbs) (Fig 4b), allowing researchers to differentiate between variants for further development. Eliminating the least stable variants allows preclinical researchers to focus on the most promising candidates in early development.
How do I determine which dose is the best for my antibody?
The use of antibodies has resolved many issues regarding drug toxicity. The inherent specificity of antibodies makes potential off-target effects significantly less likely, and, due to their composition, impossible to be converted to toxic catabolites. The primary sources of antibody-mediated toxicity are linked to the expression of the antigen at non-therapeutic sites and hyperactivation of the immune system, in the case of immunomodulatory antibodies (Brennan et al., 2010). In both cases, the appropriate dosing of the therapeutic antibody can help to prevent or limit the adverse reaction. However, if accurate preclinical data predicting the PK of an antibody in humans is not available, it is challenging to set a therapeutic dose when designing a clinical study. In many cases, it is necessary to perform a dose escalation in humans, with the risk of reducing the efficacy of the treatment or increasing the likelihood of side effects.
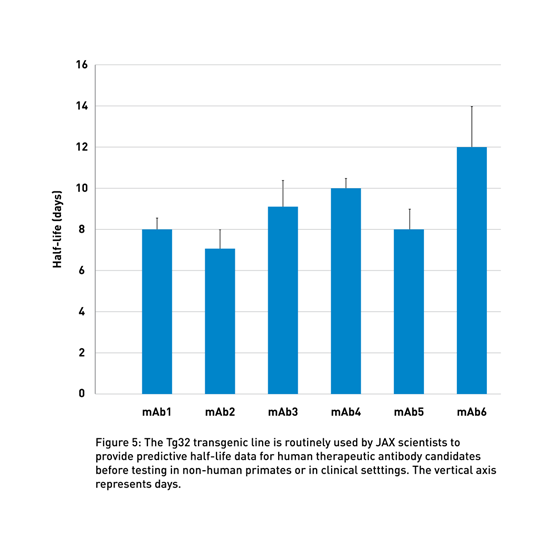
JAX-executed studies provide an accurate preclinical evaluation of antibody half-life using human FcRn Tg32 mice (014565), which display a physiological human FCGRT expression pattern (Latvala et al., 2017). These data can support decisions regarding the clinical dosing of the therapeutic molecule (Valente D, 2020; Fig 5). Using this model and human PK predictive allometric scaling (Betts et al., 2018), clinicians can estimate the minimum dose to achieve therapeutic serum concentrations, reducing the need for potentially risky dose-escalation treatments during clinical trials, as described in the presentations by Drs. Delphine Valente of Sanofi and Michael Otteneder of Roche.
What if my therapeutic generates anti-drug antibodies?
FcRn humanized mice have a functional mouse immune system. Although this is usually not an issue, sometimes human antibodies are immunogenic in rodents and elicit an anti-drug antibody (ADA) response. When this happens, the half-life of the human antibody is severely reduced, and the PK data can be challenging to interpret. If ADA manifests only in a small number of mice, it might be possible to discard the data from the affected animals.
In some cases, however, a majority of animals in an experimental group exhibit an ADA response, which undermines the entire experiment.
Scientists at JAX have backcrossed the Prkdcscid gene onto the Tg32 mouse to make it immunodeficient (018441). Since the Prkdc gene is required for generating mature B cells, mice with the scid mutation cannot produce antibodies, excluding the possibility of an ADA response (Myzithras et al., 2017; Fig 6).

Why Use JAX Antibody Testing Services?
In vitro experiments in rodents can provide some useful information about the stability of a mAb, but they cannot predict its in vivo half-life accurately. Due to inherent binding differences between the mouse and human system of IgG recycling, mAb half-life data obtained in standard mice are not translatable to humans. Until now, the only animal models that have been proven clinically predictive of mAb half-life are non-human primates. However, the use of non-human primates for antibody optimization is very problematic due to the many ethical and budgetary issues.
Dr. Derry Roopenian at JAX, recognizing this hurdle in therapeutic mAb development, has created several humanized mouse models that can be used to obtain clinically translatable therapeutic human antibody half-life data (Roopenian DC et al., 2010; Proetzel and Roopenian, 2014). JAX Therapeutic Antibody Evaluation Services are based on the use of this unique collection of innovative mouse strains and include study directors, some of whom have been directly involved in the development of the humanized mouse models used in these studies. This exclusive service from JAX is a fast and cost-effective option for researchers developing novel IgG, Fc-, or albumin-based biologics to obtain accurate, translationally relevant half-life data, and to identify the therapeutic molecules with the highest likelihood of success in the clinical setting.
To learn more, visit the JAX Therapeutic Antibody Evaluation Services page.
Recommended Resources
- JAX Therapeutic Antibody Evaluation Services
- Blog: Quick Facts to Improve Antibody Half-Life Measurements
- Blog: Revealing Antibody Half-Life and Stability Using FcRn Mouse Models from JAX
- Webinar: De-Risk Therapeutic Antibody Drug Development Using Humanized FcRn Mice
References
Avery LB, Wang M, Kavosi MS, Joyce A, Kurz JC, Fan Y, Dowty ME, Zhang M, Zhang Y, Cheng A, Hua F, Jones HM, Neubert H, Polzer RJ, O’Hara DM. Utility of a human FcRn transgenic mouse model in drug discovery for early assessment and prediction of human pharmacokinetics of monoclonal antibodies. Mabs. 8(6):1064-78. PMID: 27232760. DOI: 10.1080/19420862.2016.1193660.
Betts A, Keunecke A, van Steeg TJ, van der Graaf PH, Avery LB, Jones H, Berkhout J. 2018 Linear pharmacokinetic parameters for monoclonal antibodies are similar within a species and across different pharmacological targets: A comparison between human, cynomolgus monkey and hFcRn Tg32 transgenic mouse using a population-modeling approach. MAbs. 10(5):751-764. PMID: 29634430 DOI: 10.1080/19420862.2018.1462429.
Booth BJ, Ramakrishnan B, Narayan K, Wollacott AM, Babcock GJ, Shriver Z, Viswanathan K. 2018. Extending human IgG half-life using structure-guided design. MAbs. 10(7): 1098-1110. PMID: 29947573 DOI: 10.1080/19420862.2018.1490119.
Brennan FR, Morton LD, Spindeldreher S, Kiessling A, Allenspach R, Hey A, Muller PY, Frings W, Sims J. 2010. Safety and immunotoxicity assessment of immunomodulatory monoclonal antibodies. MAbs. 2(3):233-55. PMID: 20421713. DOI: 10.4161/mabs.2.3.11782
Challa DK, Velmurugan R, Ober RJ, Sally Ward E. 2014. FcRn: from molecular interactions to regulation of IgG pharmacokinetics and functions. Curr Top Microbiol Immunol. 382:249-72. PMID: 25116104 DOI: 10.1007/978-3-319-07911-0_12.
Latvala S, Jacobsen B, Otteneder MB, Herrmann A, Kronenberg S. 2017. Distribution of FcRn Across Species and Tissues. J Histochem Cytochem. 65(6):321-333. PMID: 28402755 DOI: 10.1369/0022155417705095
Myzithras M, Bigwarfe T, Li H, Waltz E, Ahlberg J, Giragossian C, Roberts S. 2016 Utility of immunodeficient mouse models for characterizing the preclinical pharmacokinetics of immunogenic antibody therapeutics. MAbs 8(8):1606-1611. PMID: 27598372 DOI: 10.1080/19420862.2016.1229721
Ober RJ, Radu CG, Ghetie V, Ward ES. 2001. Differences in promiscuity for antibody-FcRn interactions across species: implications for therapeutic antibodies. Int Immunol. 13(12):1551-9. PMID: 11717196 DOI: 10.1093/intimm/13.12.1551
Proetzel G, Roopenian DC. 2014. Humanized FcRn mouse models for evaluating pharmacokinetics of human IgG antibodies. Methods. 65(1):148-53. PMID: 23867339 DOI: 10.1016/j.ymeth.2013.07.005.
Roopenian DC, Christianson GJ, Sproule TJ. Human FcRn transgenic mice for pharmacokinetic evaluation of therapeutic antibodies. Methods Mol Biol 2010; 602:93–104 PMID: 27150086 DOI: 10.1007/978-1-4939-3661-8_6
Roopenian DC, Christianson GJ, Sproule TJ, Brown AC, Akilesh S, Jung N, Petkova S, Avanessian L, Choi EY, Shaffer DJ, Eden PA, Anderson CL. 2003. The MHC class I-like IgG receptor controls perinatal IgG transport, IgG homeostasis, and fate of IgG-Fc-coupled drugs. J Immunol. 170(7):3528-33. PMID: 12646614 DOI: 10.4049/jimmunol.170.7.3528
Singh S, Kumar NK, Dwiwedi P, Charan J, Kaur R, Sidhu P, Chugh VK. 2018 Monoclonal Antibodies: A Review. Curr Clin Pharmacol. 13(2):85-99. PMID: 28799485Wang X, Mathieu M, Brezski RJ. 2018. IgG Fc engineering to modulate antibody effector functions. Protein Cell. 2018 Jan;9(1):63-73. PMID: 28986820 DOI: 10.1007/s13238-017-0473-8.
Ward ES, Devanaboyina SC, Ober RJ.2015. Targeting FcRn for the modulation of antibody dynamics. Mol Immunol. 2015 Oct;67(2 Pt A):131-41. PMID: 25766596 DOI: 10.1016/j.molimm.2015.02.007.
Valente D, Mauriac C, Schmid T, Focken I, Beninga J, Mackness B, Qiu H, Vicat P, Kandira A, Radošević K, Rao S, Darbzshire J, Kabiri M. Pharmacokinetics of novel Fc-engineered monoclonal and multispecific antibodies in cynomolgus monkeys and humanized FcRn transgenic mouse models. MAbs. Jan-Dec 2020;12(1):1829337. PMID: 33079615. DOI: 10.1080/19420862.2020.1829337.
Wang X, Mathieu M, Brezski RJ. 2018. IgG Fc engineering to modulate antibody effector functions. Protein Cell. 2018 Jan;9(1):63-73. PMID: 28986820 DOI: 10.1007/s13238-017-0473-8.
Ward ES, Devanaboyina SC, Ober RJ.2015. Targeting FcRn for the modulation of antibody dynamics. Mol Immunol. 2015 Oct;67(2 Pt A):131-41. PMID: 25766596 DOI: 10.1016/j.molimm.2015.02.007.