Experimental design: Top four strategies for reproducible mouse research

Figure 1. These mice may be genetically identical siblings, but each is a unique, independent variable in your experiments.
Famous last words
We’ve all been there before:
“I just need one more replicate experiment and then this paper is done!”
Oh, how the mighty have fallen.
While your goal as a biomedical researcher may be to test your hypothesis in a living organism, a mouse’s goal is simple: be a mouse. Unlike a chemical reagent or even an immortalized cell line, inbred mice are biological entities that, despite being more-or-less genetically identical within a particular strain, can show phenotypic variability, are sensitive to small environmental insults, and continue to change developmentally as days and weeks pass. This is why laboratory mice need special consideration from researchers and animal care staff to promote reproducible research.
Design mouse studies with the 3Rs in mind
The National Centre for the Replacement, Refinement and Reduction of Animals in Research (NC3Rs) developed principles for humane animal research over 50 years ago. These guidelines not only provide a framework for ethical animal use, but also promote robust and reproducible animal research through careful and thoughtful experimental design.
Because The Jackson Laboratory has been maintaining mouse strains for over 80 years, we are well-acquainted with the intricacies involved in designing mouse experiments. From careful selection of one or more of the over 8000 unique mouse strains in our repository to testing enough mice to adequately power your experiments, the following key considerations fall within the context of the 3Rs and promote the “4th R” – Reproducibility.
1. Know thy mouse and choose wisely
Mouse strains are as variable as dog breeds, if not more so. Golden Retrievers and Chihuahuas are both dogs, but each has specific characteristics that make them unique – appearance, demeanor, and propensities to develop certain health issues, to name a few. Mice are no different. Laboratory mice vary widely in appearance (from the hairless nudes to the black and tan BTBRs shown below), demeanor (from the feisty BALB/cJ males to the more docile A/J mice), and in their propensities to develop certain health issues (from leukemia in AKR to retinal degeneration in FVB mice), sometimes making them suitable models for human diseases and sometimes making them a challenge to use experimentally.


Figure 2. Not all mice are created equal. Nude mice (left) are immunodeficient and hairless, making them useful in cancer research. BTBR mice (right) exhibit several behavioral phenotypes useful to investigate certain aspects of autism.
Peer-reviewed, published literature should be a primary method for choosing mouse strains that make sense for your scientific question: the more publications that exist for a strain, the more likely it is that characterization has already been done, and the more support you will have when analyzing your results.
However, keep in mind that some publications may use certain strains for historical rather than biological reasons. Therefore, the Mouse Phenome Database (MPD) can be a rational supplementary method to choose an appropriate model. As a user-submitted database that collects comprehensive strain characteristics for many commonly used inbred mice and some data from a few commonly used mutant models, MPD may help you to find a strain that may not have been used in say, cardiovascular research, but has a naturally high blood pressure in which you could test an experimental compound or knock out a gene to study its effects on blood pressure.
Once you have narrowed your choices to a few strains, ask yourself these kinds of questions and refine your selections as needed:
- Given your hypothesis, does the mouse model make sense? For example, if you are modeling a human disease, how does the mechanism of disease development compare between the mouse and human?
- Does the desired phenotype vary with the sex, age, or genetic background (including substrain)?
- How might the animals’ housing conditions, health status, or their microbiome affect the phenotype?
- What are the limitations to using this strain? Might you need multiple models to completely answer your question (see some examples in spinal muscular atrophy)? Given the model’s characteristics, are there undesirable phenotypes like audio/visual impairments, tumor incidence, or fertility issues that might interfere with your experimental goals?
2. Implement handling practices and design experimental procedures with the mouse in mind
Once you have selected the strain or strains that you plan to use, keep in mind that mice are affected by what is going on around them. It is important to be aware that how you handle the mice may have experimental consequences. Design your study to minimize or account for these consequences.
Adopt best colony management practices
How you manage your mouse colony is the key to maintaining genetic stability and reproducibility. Keep these considerations in mind as you expand and contract your colony:
- Maintain a defined breeding rotation and mating scheme to avoid accidentally selecting for abnormally mild or strong phenotypes
- Keep pedigrees and excellent records, removing any phenotypic deviants from the colony
- Refresh genetic backgrounds regularly by backcrossing your mice every 5-10 generations to the appropriate inbred, hybrid, or outbred strain
- Cryopreserve unique strains so you can recover mice with the original phenotype and genetic background, if necessary
- Purchase experimental mice directly from a trusted vendor who implements measures that limit genetic drift and has strict quality control measures in place to maintain a consistent mouse model
Avoid exposing laboratory mice to unnecessary stress
Much like you and me, when mice are stressed they may not behave as they would under non-stressed conditions. The “simple” act of handling mice may exacerbate or minimize a phenotype, making reproducibility a challenge. For a strain such as the mdx mouse, a model of muscular dystrophy, escape behaviors by the mouse and handling by the experimenter could be detrimental to mouse muscle tone and may affect assay readouts. Mice used for metabolic disorders may exhibit unstable blood glucose values when prodded too frequently, lose weight with overhandling, or vary in phenotypic onset when housed alongside environmental stressors. Finally, pheromones secreted by men have been shown to increase stress hormones and influence pain-related behaviors in mice.
Can you account for potential sources of stress in housing or experimental design and what might you be able to do to mitigate them?
Select assays wisely
Mice do not always “behave.” While you may be planning to test your mice on a rotarod to evaluate balance or grip strength, a mouse may never learn to walk on it or may learn to jump off. For any given assay, there are limitations and caveats to data interpretation that should be considered carefully. Additionally, you may need to plan to have multiple methods to test your hypothesis or determine whether your intervention worked.
Avoid bias
Further, you should practice blinding and test subject randomization to ensure that experimenter bias is removed, especially when scoring assays that may have somewhat subjective measures. Enlist a trusted labmate to blind and randomize your studies while you focus on running your assays. Randomization may take many forms including: mouse sex, distributing experimental groups across multiple cages and litters, and the location of the mouse cages in the room or on the racks.
While not all of these contributions to variability can be avoided, you can record deviations in your protocol, minimize experimenter or handler turnover, or note behavioral observations throughout the course of your experiments to account for potential variability. Mice, like a micromanaging PI, may notice everything you do in the lab with effects on reproducibility. Learning to account for the sources of such variability is the key to understanding your data.
3. Perform pilot studies and do the math
Once you understand how your mouse is supposed to behave and measures you can take to keep your experiments as consistent as possible, it’s time to get started. It is tempting to put all of your eggs in one basket and perform a full-blown experiment on your first try. However, if you are unfamiliar with the mouse model or an assay, it is highly advisable that you run a pilot study followed by rigorous statistical analysis including p-value, effect size and experimental power. Pilot studies may reveal several things:
- How the mouse phenotype manifests itself in your hands with your assay, and how it compares to published literature
- Whether you need to include more mice to have an appropriately powered study
- Whether you should consider other assays that have more robust effects
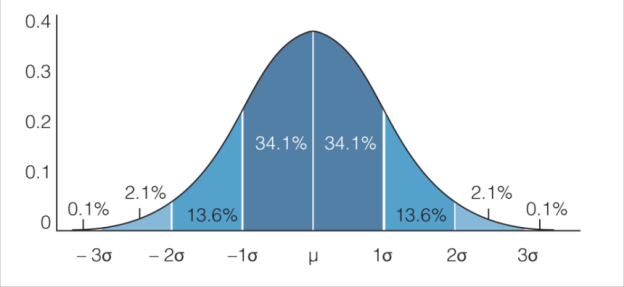
Remember, the prevalence and intensity of desired (and undesired phenotypes) in mouse strains will follow a normal distribution, such that 95% of all mice will fall within 2 standard deviations of a given mean. Additionally some mice may become ill, may die, or may be lost to equipment failures before completing your study. Therefore, it is wise to include a few extra mice in each study cohort beyond the sample size you calculated from your pilot study and power analysis.
4. Report completely and accurately
Once you have successfully discovered a new biological truth, you may be eager to publish. Reproducibility in mouse research relies on responsibly reporting your methods in publications. Key details you should communicate in your publication include descriptions of mouse age, sex, and number, complete nomenclature, housing conditions, and appropriate statistical analyses. In addition, the NC3Rs suggests several other experimental details to include in publications and have systematically presented them in their Animal Research: Reporting of In Vivo Experiments (ARRIVE) Guidelines publication. Likewise, the National Institutes of Health (NIH) also offers guidelines for Rigor and Reproducibility in Grant Applications, which should apply to publishing your data, too.
With careful model selection, practices to stabilize phenotype, rigorous statistical analysis, and sufficient sample sizes, your research using mice can serve as the foundation for future discoveries and novel therapeutic approaches.