Nude Mice - More than What Meets the Naked Eye
The mouse is arguably the most widely used mammalian model in biomedical research. Their relatively small size, and both genetic and physiological similarity to humans, make mice a model organism for human disease. It was in the 1960s that the first publication specifically mentioned nude mice, also known as athymic mice. This is a particular mouse strain that has a mostly “hairless” phenotype (Pantelouris 1968; Mecklenburg L et al., 2004). In the 50-plus years since the discovery of nude mice, the field has changed significantly due to the development of gene-targeting technology. But even through the years, nude mice remain just as relevant today as they were then in a variety of therapeutic areas, including oncology and immunology.
So if you have a colleague who’s using nude mice, or you are starting to use them yourself, here is some background information on these models and some tips on handling them.
What are Nude Mice?
Nude mice are an invaluable tool in biomedical research and drug discovery. Due to their immuno-comprised phenotype, they have proven to be useful for xenografts, particularly in cancer research (Wettersten, Ganti, & Weiss, 2014; Szadvar, I et al., 2016). Thus, nude mice lack a proper thymus, T cell maturation, and may show small amounts of sparse, generally abnormal fur or whiskers that are structurally weak and break as they grow (Mecklenburg et al., 2004).
Being athymic, they do not produce mature T cells and exhibit a reduced number of circulating lymphocytes, as compared to wildtype or heterozygous controls. This also results in a lack of antibody production (Muqbil, Philip, & Mohammad, 2019; Wettersten et al., 2014). These phenotypes are useful as it allows a range of foreign cells or tissue to engraft into the mouse host.
Taken together, these characteristics of the nude mice make them an excellent model for xenografting tumor cell lines, understanding mechanisms of tumor malignancy, and testing the impact of potential treatments (Price, 2001).
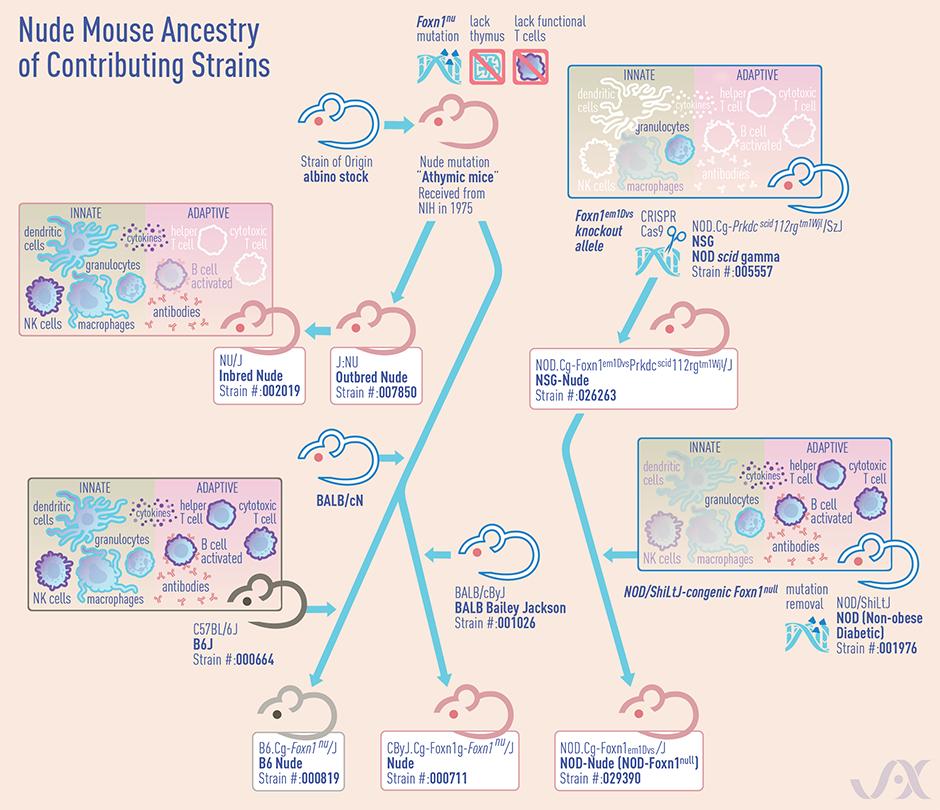
What nude mouse strains exist, and how do they differ?
In 1975, The Jackson Laboratory (JAX) imported the Foxn1nu mutation from the National Institutes of Health (NIH) on an outbred stock of mice. Today, JAX now maintains several nude strains from NIH. Here’s some information on the strains most often used by researchers:
Inbred nude mice: NU/J (002019)
By definition, an inbred strain is produced using matings between brother and sister or parent and offspring, for at least 20 generations. As noted in the datastrain sheet, these NU/J inbred mice have a homogenous mouse genetic background, which allows for highly consistent and reproducible growth of many allogeneic cell lines.
Outbred nude mice: J: NU (007850)
This strain is maintained using a highly specialized outbred mating scheme, which avoids mating between closely related individuals. By avoiding these crosses, this strain can retain greater genetic diversity and improve animal vigor. As these mice are more genetically heterogeneous, there may be greater mouse-to-mouse phenotypic variability than inbred mice. However, these mice may breed better, live longer, and may be better able to withstand surgical procedures and other experimental manipulation (Zeineldin M et al., 2014).
B6.Cg- Foxn1nu/J (000819)
These mice have the Foxn1nu mutation fully congenic on the C57BL/6J inbred genetic background. They may be useful in the adoptive transfer of C57BL/6-derived immune cells and genetic crosses with other genetic mutants with the same genetic background.
For more information about the development of these strains, be sure to review the strain datasheets for each specific model, under “Details, Detailed Descriptions.”
Handling Nude Mice
Mouse health directly correlates with data integrity and scientific reproducibility. Nude mice do require additional precautions as they are immunodeficient. Here are some tips for caring for these mice.
- Wear Personal Protective Equipment (PPE) to help protect the mouse from getting infections from humans. It also protects those working in the facility against bites and allergies. Disposable PPE should be changed with every entry to the vivarium and the dedicated equipment should be carefully stored in the vivarium to minimize exposure to contaminants.
- Keep handling to a minimum. While it’s essential to check on these mice regularly, over-handling can be disturbing to mice and also increases the chances of introducing pathogens to their environment.
- Choosing Housing and environment carefully. Be aware that the type of housing can have a significant effect on managing an immunodeficient colony. Factors, such as those that limit the flow of researchers and workers, including an entryway to the vivarium with limited entry, clear signage, and possibly changing the cages more often, may limit the likelihood of infection derived from gut flora present in the mouse species passing from humans to mice. For more information on this, be sure to look at this webinar.
- Utilize a cleanroom.A cleanroom of immunodeficient mice is a happy room. This can be achieved by using an effective sanitization program, which includes regular cage cleaning, room cleaning, disinfecting surfaces, and sanitization processes.
- Practice aseptic techniques when working with these mice. As cleanliness can only go so far, there will be microorganisms present in any vivarium. Aseptic techniques are designed to minimize potential contamination, which means establishing training and retraining programs with verification steps to ensure that proper care is taken to reduce exposure to the immunodeficient strains.
For 90 years, JAX is committed to presenting the global research community with up-to-date information about best practices to ensure the safety and health of laboratory mice. With bio-security measures and rigorous testing, JAX strives to maintain a high health status to all of the mouse models under our care, including our nude and other immunocompromised mouse strains. To learn more, check out the JAX health status reports available, in addition to the nude mice strains available from JAX.
Recommended Resources
Webinar: Caring for Immunodeficient mice — from Nude to NSG™
References
Mecklenburg, L., et al. (2004). "FOXN1 is critical for onycholemmal terminal differentiation in nude (Foxn1) mice." J Invest Dermatol 123(6): 1001-1011.
Muqbil, I., et al. (2019). Chapter 9 - A guide to tumor assessment methodologies in cancer drug discovery. Animal Models in Cancer Drug Discovery. A. Azmi and R. M. Mohammad, Academic Press: 233-248.
Pantelouris, E. M. (1968). "Absence of thymus in a mouse mutant." Nature 217(5126): 370-371.
Price, J. E. (2001). "Xenograft models in immunodeficient animals: I. Nude mice: spontaneous and experimental metastasis models." Methods Mol Med 58: 205-213.
Wettersten, H. I., et al. (2014). "Metabolomic profiling of tumor-bearing mice." Methods Enzymol 543: 275-296.
Szadvari, I., et al., (2016). “Athymic Nude Mice as an Experimental Model for Cancer Treatment.” Physiological Research 65 (Supplement 4): S441-S45.
Zeineldin, M., et al. (2014). "Human cancer xenografts in outbred nude mice can be confounded by polymorphisms in a modifier of tumorigenesis." Genetics 197(4): 1365-1376.