5 Common Questions for Diabetic Models
In a previous blog post, we discussed some important Do’s and Don’ts of working with diabetic mouse models. Here we will discuss some of the most common questions we get from investigators who are working with these models.
1. What is the BEST model to use for diabetes research?
The best model will really depend on which specific characteristic of diabetes you plan to study. Are you looking for a model with the highest possible blood glucose values? Or are you hoping to study the role of your drug in hyperinsulinemia? There are so many options, and one model may not cover all of the characteristics that you want to study. You might consider running your studies in multiple models, to determine if your research is more translatable.
There are already several resources that have been compiled to help you find the models that might help achieve your research goals, such as this comparison chart and this blog post of type 2 models. Searching through the current literature in your field will also reveal if any new models have been generated, or if new information has been reported on some of the more common strains in use.
Are you looking at these models and realizing that you actually want a model of type 1 diabetes, where the pancreatic beta-cells are depleted? There are inbred strains, such as the NOD/ShiLtJ, that spontaneously develop diabetes. If you would like to induce pancreatic beta-cell death, there are also some chemical options available – one common option is treatment with streptozotocin (STZ). Steptozotocin is a powerful chemical that mimics glucose structure closely enough to be transported into the beta-cell via GLUT2 – but once inside, wreaks havoc in the form of DNA damage and PARP activation. A high percentage of the beta-cells are destroyed, leaving the pancreas no way to produce insulin, hence the diabetic phenotype. JAX offers STZ services for the C57BL/6J, NOD.CB17-Prkdcscid/J, and NSG mouse models.
2. Do I have to fast my mice before I measure their blood glucose values?
Not necessarily! Looking at both fed and fasting glucose levels can give you great information – depending on what you are looking for.

Fed glucose levels, or mice that have had free access to the food in their cage prior to taking the measurement, can provide information on circulating levels of glucose, generally coming from the diet. This can be a good way to look at overall metabolism in the mouse – you haven’t stressed the system with a fast. The data you obtain from a cohort of fed mice might be pretty variable, as you haven’t controlled for when they last ate, how much they ate, and how active they have been. One mouse in your group may have eaten in the last 20 minutes, while another mouse in the same cohort hasn’t had food in a few hours!
Fasted glucose levels tend to provide less variable results, as you removed the food source for a designated amount of time for all of the mice involved. The current glucose levels in the blood after a fast will be regulated by the metabolic processes of gluconeogenesis and glycogenolysis, and there is a ‘normal’ range that these levels are likely to be in, depending on how long the fast is. For ‘healthy’, non-diabetic mice, a fasting blood glucose level of 80-100 mg/dL is often measured after a 4 – 6 hour fast.
For both fed and fasting measurements, the important part is to ensure you are consistent with the time of day you are measuring blood glucose levels for all mice in the experiment.
Fasting mice – this could be its own book! But for the purposes of brevity, we will just go through a quick point: Why is it so important to keep the time of day you are measuring blood glucose consistent if you are fasting your mice? Shouldn’t a 4 hour fast between 8 am – noon give the same results as a fast from noon – 4 pm? Mice are nocturnal, and consume about 2/3 of their food during the dark cycle. If you arrive at 8am to take fed blood glucose values, there is a really good chance those mice have been eating within the last hour. However, if you take your fed blood glucose measurements at 4pm, the resulting values may be a bit closer to a fasted state.
Along the same line, a mouse fasted from 8 am – noon is likely a true 4 hour fast, while the mouse fasted from noon – 4pm might be closer to an 8 hour fast as the mice were likely asleep between 8 am-noon! This can result in wildly different measurements (depending on what you are looking at – while blood glucose may not be significantly different in a 4 or 8 hour fast, there are some liver values that may give very different results based on fasting time).
Not all fasts are the same! A 5 hour fast might result in a 5% loss of body weight while an 18 hour fast can result in as much as a 16% loss in body weight (Ayalya 2006). An 18 hour fast may seem like the best idea, as you can take out their food before you leave work, and perform the test in the morning. But as mice eat the majority of their food during the night, this ’18 hour fast’ might more similarly model a 24 hour fast (the mice have been sleeping all day, not eating!).
And be mindful! Mice are known to grind some of their food – leaving bits mixed up in their bedding. If you are looking to truly fast your mice you may have to move them into a new, clean cage with no food hopper. Simply pulling out their hopper will actually leave the hungry mice to dig through their bedding and eat any crumbs they can find – confounding your data collection.
3. Does it matter how I test – using a glucometer or a biochemical assay?
This is a hotly debated topic throughout the metabolism field – what is the best way to read your glucose results? Some investigators swear by mouse-specific glucometers, while others purchase patient-approved glucometers off Amazon because they are much less expensive. Still others insist that a biochemical assay is the only way to go.
Consistency will be the most important way to obtain usable, reproducible data. Determine which method will work best in your lab with the resources you have available and continue to use the same method throughout the course of your entire study. There have been many publications evaluating the accuracy of different methods (Morley 2018, Togashi 2016), which might help you decide which to choose.
If using a glucometer, there are some important steps that need to be taken before you begin to use this on your mice. To reduce variability, have a dedicated glucometer for your mouse studies. This will help prevent any machine-to-machine variability that is common with any electronic apparatus being used.
Calibration is required for every device, and often a set of standards will come with your glucometer. Follow the manufacturer’s instructions exactly as they have described them – including re-calibrating the machine on a regular basis. When was the last time you calibrated your glucometer?
One common issue with glucometers is the detection limit. Many glucometers will have a limit of 500 mg/dL, and any sample higher than that will simply read as ‘hi’. Depending on which mouse model you are working with this could be a huge problem! Certain diabetic mouse strains might push this limit, such as the BKS.Cg-Dock7m +/+ Leprdb/J and KK.Cg-Ay/J. If you are working with a strain that may not be detectable by glucometer, you may have to consider other options such as a biochemical assay.
Biochemical assays, such as glucose oxidase assays, are another common way to measure blood glucose from your mice. There are many such colorimetric or fluorometric assays that are coupled with enzymes to cause a colormetric change that can be measured spectroscopically. These assays do not have as low of a detection limit as a glucometer, as you depend on a standard curve run with each assay to determine your results, allowing for much higher glucose concentrations.
And finally, always sample blood from the same site. There are many publications that have shown glucose results may be different based upon the location of the blood draw (Rogers 1999).
In the image below, JAX performed validation studies using both a OneTouch glucometer using whole blood, to a chemistry analyzer using plasma. Consistently, plasma glucose levels were 5% greater, on average, than whole blood. This highlights the need to use the same method consistently in your mice while on study.
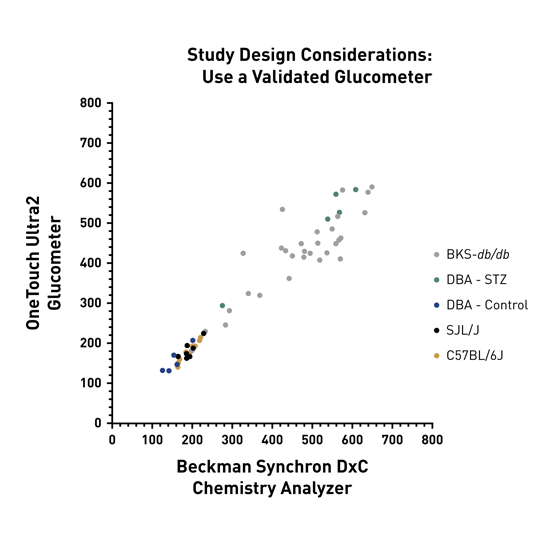
Validated Glucometer Image Caption: Glucose measurements were highly correlated between the OneTouch glucometer (whole blood) and chemistry analyzer (plasma) (r = 0.966, n = 57, p < 0.0001).
4. Why aren’t my mice breeding?
There are a lot of factors that affect breeding performance, and this is particularly true in the case of diabetic mouse models. We know that environmental conditions, stress, and sometimes dietary fat content can all help or hurt the chances of obtaining litters (here are some helpful tips on how to help!). For diabetic models, certain genotypes of mice are simply not capable of breeding. Both the ob/ob and db/db mouse models are infertile due to perturbations in the leptin signaling pathway; these strains must be maintained by breeding together heterozygous mice to obtain the homozygous, diabetic mouse model. Strategies such as ovarian transplant may be considered, such as in the case of the ob/ob mutation – the oocyte itself is capable of producing offspring, but peripheral factors in the diseased mouse lead to infertility. Transplanting a homozygous ovary into a wild type female can be used to build your colony, which is how JAX maintains these mice.
Aside from the common ob/ob and db/db models, many other mouse models of diabetes have trouble breeding. This can often be attributed to high blood glucose levels leading to lethargic, sick mice. Sick mice may not be physiologically infertile, but that doesn’t mean they are going to breed.

Some models, like the NOD/ShiLtJ mentioned earlier, can be treated with compounds to help increase breeding performance. By subjecting these mice to an early immune challenge, such as injecting the mice with Complete Freund’s Adjuvant, the onset of the disease is actually slowed down or prevented altogether (Sadelain 1990). This allows the females to have more litters successfully than untreated females, helping maintain the colony.
The Akita model is another example of a strain that has a shortened breeding span due to the diabetic phenotype of the male mouse. KKAy mice are fertile at younger ages, but as the disease progresses these mice become infertile. The phenotype and disease progression can play a large role in breeding success of your mouse models. It is important to read the literature on your model of interest to determine if there are any special breeding characteristics associated with a strain before you begin your colony, to increase your chances of success.
5. Why aren’t my mice diabetic?
Checking the genotype of the mice will be the first step in troubleshooting. Did you receive heterozygous ob mice instead of homozygous?
Unfortunately, sometimes mice do not become diabetic despite having the correct genotype or food provided. Some disease models do not have 100% penetrance, or may be sex-dependent: the NOD/ShiLtJ strain is a great example of both of these traits. Only about 55% of male mice will become diabetic by 30 weeks of age. If you are planning a study and need diabetic 15 week old male mice, you can only expect about 5% of the males to be diabetic at this timepoint. Adjusting your cohort numbers appropriately before you begin your study will be critical to ensure you build in these buffer zones based upon the known disease progression in this model. Conversely, 90% of female mice become diabetic by 30 weeks of age – though you should still look at the published literature for the specific time point you are planning your study.
NONcNZO10/LtJ mice only exhibit 85% penetrance by 18 weeks of age, and this is closely tied to the diet these mice are fed. Penetrance is much higher in mice fed a higher fat diet compared to standard chow (discussed in more detail here). In TALLYHO/JngJ mouse colonies, both the males and females develop obesity but only the males exhibit the hyperglycemia phenotype.
These diabetic phenotypes may also be dependent on stress. A model that relies on weight gain to obtain the diabetic phenotype may not be eating if they are experiencing stress within the colony, altering disease onset. You can help reduce stress in your colony by following some breeding tips we provide for all mouse colonies.
Diabetic mouse models can be challenging to work with, and require special care and planning. Focusing on the specific characteristics or phenotype you are interesting in studying can help determine which model might best suit your research. As always – please feel free to reach out to the Technical Information Services team at JAX with any other questions you might have!
Resources:
References:
Ayala, J.E., Bracy, D.P., McGuinness, O.P., Wasserman, D.H., 2006. Considerations in the design of hyperinsulinemic-euglycemic clamps in the conscious mouse. Diabetes 55, 390–397.
Grasemann, C., Devlin, M.J., Rzeczkowska, P.A., Herrmann, R., Horsthemke, B., Hauffa, B.P., Grynpas, M., Alm, C., Bouxsein, M.L., Palmert, M.R., 2012. Parental Diabetes: The Akita Mouse as a Model of the Effects of Maternal and Paternal Hyperglycemia in Wildtype Offspring. PLOS ONE 7, e50210.
Morley, L.A., Gomez, T.H., Goldman, J.L., Flores, R., Robinson, M.A., 2018. Accuracy of 5 Point-of-Care Glucometers in C57BL/6J Mice. J Am Assoc Lab Anim Sci 57, 44–50.
Rogers, I.T., Holder, D.J., McPherson, H.E., Acker, W.R., Brown, E.G., Washington, M.V., Motzel, S.L., Klein, H.J., 1999. Influence of Blood Collection Sites on Plasma Glucose and Insulin Concentration in Conscious C57BL/6 Mice. Contemp Top Lab Anim Sci 38, 25–28.
Sadelain, M.W., Qin, H.Y., Lauzon, J., Singh, B., 1990. Prevention of type I diabetes in NOD mice by adjuvant immunotherapy. Diabetes 39, 583–589. https://doi.org/10.2337/diab.39.5.583
Togashi, Y., Shirakawa, J., Okuyama, T., Yamazaki, S., Kyohara, M., Miyazawa, A., Suzuki, T., Hamada, M., Terauchi, Y., 2016. Evaluation of the appropriateness of using glucometers for measuring the blood glucose levels in mice. Sci Rep 6, 25465. https://doi.org/10.1038/srep25465