Redefining Preclinical Gene Delivery Testing: Humanized Mouse Models as an Ethical and Effective Alternative to NHPs
As gene therapy continues to revolutionize modern medicine, the demand for reliable, translational, and ethically sound preclinical models has never been higher. In vivo gene delivery systems, particularly those aimed at generating CAR cells, are at the forefront of this transformation. However, their development faces significant challenges, including low transduction efficiency, immunogenicity of viral vectors, and off-target effects that can compromise both safety and efficacy.
Historically, non-human primates (NHPs) have been the preferred model for testing these systems due to their similarity to humans. However, growing ethical concerns, high costs, and increasing regulatory restrictions, such as the FDA's roadmap to phase out NHP use, have driven the search for more sustainable and human-relevant alternatives.
The NSG SGM3xIL15 and NSG FLT3xIL15 Humanized Mouse Models
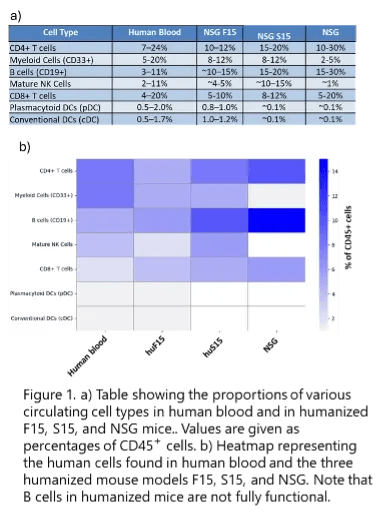
Developed by JAX, these next-generation humanized mouse models offer a powerful solution. While the first humanized model on the market, the NSG, supported only limited lineage differentiation, the third-generation humanized models have been engineered to express human cytokines such as IL-15, IL-3, FLT3L, and GM-CSF, and support robust multilineage hematopoiesis and immune system reconstitution (see Figure 1 for a comparison with the NSG models). The immune system in hematopoietic stem cell reconstituted NSG SGM3xIL15 (S15) and NSG FLT3xIL15 (F15) mice includes functional T cells, NK cells, and myeloid cells, which are essential for evaluating gene delivery systems in a human-relevant context.
Unlike traditional models, such as NHPs or WT mice and rats, S15 and F15 mice provide:
A multilineage human immune system, enabling precise evaluation of vector targeting and immune responses
Reduced risk of graft-versus-host disease (GvHD) compared to PBMC-based models, allowing for longer and more stable study windows of months rather than weeks
Translational relevance that bridges the gap between rodent models and human clinical trials
Why These Models Matter for Gene Delivery
Gene delivery systems, whether viral (e.g., AAV, retroviral) or non-viral (e.g., lipid nanoparticles), must be tested in environments that accurately reflect human immune responses. The S15 and F15 models allow researchers to:
Assess transduction efficiency in a variety of human immune cells.
Monitor immune activation and cytokine release, critical for safety profiling.
Evaluate off-target effects and long-term expression of therapeutic genes.
Test novel delivery platforms, including CRISPR-based and nanoparticle-mediated systems.
These capabilities are essential for in vivo cell therapies, where precise gene delivery and immune modulation are key to therapeutic success.
Replacing NHPs: A Regulatory and Ethical Imperative
With the FDA and other regulatory bodies encouraging alternatives to NHPs, humanized mouse models are emerging as the preferred platform for in vivo preclinical gene therapy testing. They offer:
Scalability and cost-effectiveness for preclinical applications
Ethical advantages by avoiding the use of sentient primates
Regulatory alignment with evolving standards for human-relevant data.
Moreover, the small size and immediate availability of these mice make them ideal for repeated studies, accelerating the pace of innovation.
A Smarter Path Forward
The S15 and F15 humanized mouse models represent a paradigm shift in preclinical gene delivery research. By combining translational accuracy with ethical responsibility, they empower researchers to develop safer, more effective therapies—without relying on NHPs. As the field of gene therapy advances, these models will be instrumental in shaping the future of personalized medicine, immunotherapy, and other fields.