Preclinical Modeling of T Cells in Immuno-Oncology
Author: Janine Low-Marchelli, Ph.D. Senior Technical Information Scientist
While numerous immune cell types interact with tumor cells, T cells remain a primary focus for therapy. Given that mouse biology doesn’t always predictably model human biology, how are some researchers developing T cell-based immuno-therapies in the preclinical space?
Classic syngeneic tumor models allow the study of endogenous T cell function
Tumors that spontaneously arise or are experimentally induced in a mouse can sometimes be isolated and propagated in vitro. When these cell lines, such as B16F10 melanoma or CT26 colon carcinoma, are injected back into the compatible inbred mouse strain of origin (C57BL/6J or BALB/cJ in these examples), these syngeneic models allow the study of endogenous T cell function in fully immune competent mice.
-
University of Chicago researchers used the C57BL/6-derived B16F10 melanoma cell line to investigate the role of the microbiome in immunotherapy. Anti-PD-L1 therapy generated stronger tumor-specific T cell responses and intratumoral CD8+ T cell accumulation when the cells were implanted in the C57BL/6J mouse substrain from The Jackson Laboratory (JAX) compared to the C57BL/6Tac mouse substrain from Taconic Farms. Members of the genus Bifidobacterium, found in higher composition in the microbiome of the JAX mice, exerted these anti-tumor effects.
-
Mariathasan and colleagues selected the BALB/c-derived EMT6 mammary carcinoma cell line to study tumors that do not respond to anti-PD-L1 therapy. They found that co-administration of anti-PD-L1 with anti-TGFβ, which targeted tumor stromal cells, allowed for better T cell infiltration into the tumor and improved tumor regression.
Basic T cell immuno-oncology and proof-of-concept studies are possible using syngeneics, but may lack translatability to human-specific molecules. However, translatability can be improved by generating new mouse models that genetically express human-specific variant molecules in place of their mouse counterparts. This can be done relatively quickly using CRISPR/cas9 technology to make the precise edits directly in the exact mouse genetic background of interest, ensuring the matching tumor cell line will still engraft with no immune rejection (JAX Custom Model Generation Services).
Immunodeficient mice permit the study of human T cells and human cancers in vivo
To generate human-specific therapies, permissive immunodeficient mouse strains, which lack the ability to mount an immune response, can be engrafted with tissues from human donors. Some sophisticated models include co-engraftment of both human immune cells and human cancer cell lines or Patient-Derived Xenografts (PDX) (JAX Onco-Hu Models).
-
Chimeric Antigen Receptors (CAR) have been used clinically to treat leukemias with success. However, the current versions can illicit unintended inflammatory immune reactions, because they contain fragments of mouse origin. To address this, Sommermeyer and colleagues employed the severely immunodeficient NSG mouse strain to test fully human CD19 chimeric antigen receptors (CARs) against a human leukemia. They successfully showed reductions in the immunogenicity.
-
Researchers at Merck chose both syngeneic models and NSG mice humanized with CD34+ hematopoietic cells to test the efficacy of anti-GITR on a human melanoma cell line. Treatment with anti-GITR antibody reduced the Treg population and activity within the spleen and tumor, allowing CD4+ and CD8+ T cells to increase production of antitumor effector cytokines.
Immune system humanization continues to be improved with the addition of transgenic human cytokines, such as in NSG-SGM3 mice that express human IL3, CSF2, and KITLG. Additionally, when NSG mice are engineered to express human MHC molecules, such as NSG HLA-A2, and are paired with matched PDX and CD34+ cells, the humanized T cells can become appropriately MHC restricted. Depending on the application, NSG mice that lack endogenous MHC I and MHC II molecules (https://www.jax.org/strain/025216 ) can be used as hosts for human donor peripheral blood mononuclear cells (PBMC), reducing complications of Graft-vs-Host Disease (GvHD) and allowing rapid evaluation of human T-cell biology in vivo.
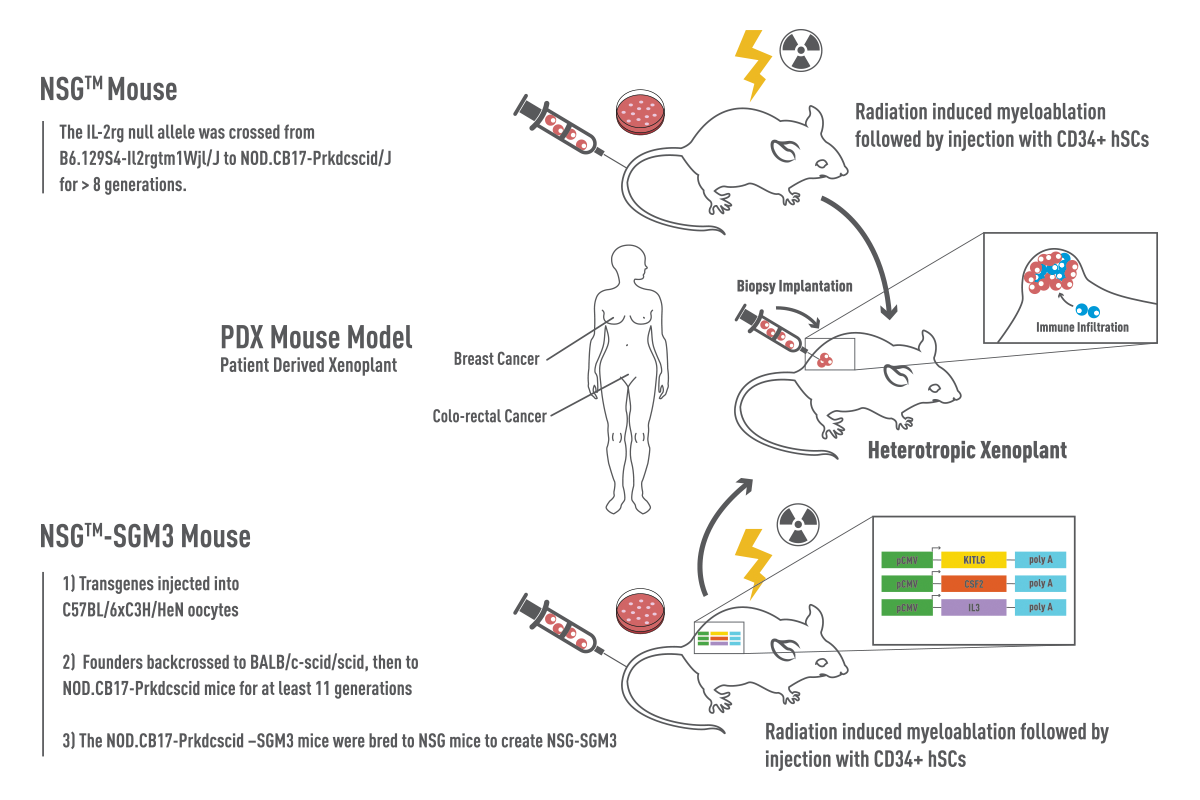
Figure 1: NSG vs NSG-SGM3 Mice. NSG™ mice (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ, 005557) are devoid of mature B, T, and NK cells. NSG™-SGM3 (NOD.Cg-Prkdcscid Il2rgtm1Wjl Tg(CMV-IL3,CSF2,KITLG)1Eav/MloySzJ, 013062) are NSG™ mice that contain transgenes expressing human Stem Cell Factor (KITLG), GM-CSF (CSF2), and IL-3. Recipient mice were irradiated at 3-4 weeks of age and engrafted with human-cord-blood derived CD34+ hematopoietic stem cells. Prior to co-engraftment of human tumors, mice are validated for human multilineage engraftment and establishment of human immunity.
Ex vivo models facilitate quick compound screening or genomics analysis
Patient-to-patient variability and intratumor variability can be modeled using clinically relevant patient-derived tumor tissues either in vivo or ex vivo. Scalability is a primary advantage of ex vivo modeling, including screening compound libraries on a large number of diverse samples. In addition, primary tissues can be cultured on a short term basis and contain autologous stroma and passenger immune cells that would be lost if the tissues were passaged in vivo. Using a bank of primary tumor tissues, genomics and bioinformatics analyses may allow broader generalizations about cancer signatures.
There is a wide variety of in vivo and ex vivo models that are helping to develop better, more targeted cancer therapies. One thing is clear though, models are approximations, and multiple models are usually needed to adequately understand and treat a disease. Certainly with a disease as complex and heterogeneous as cancer, there is no exception to this rule.